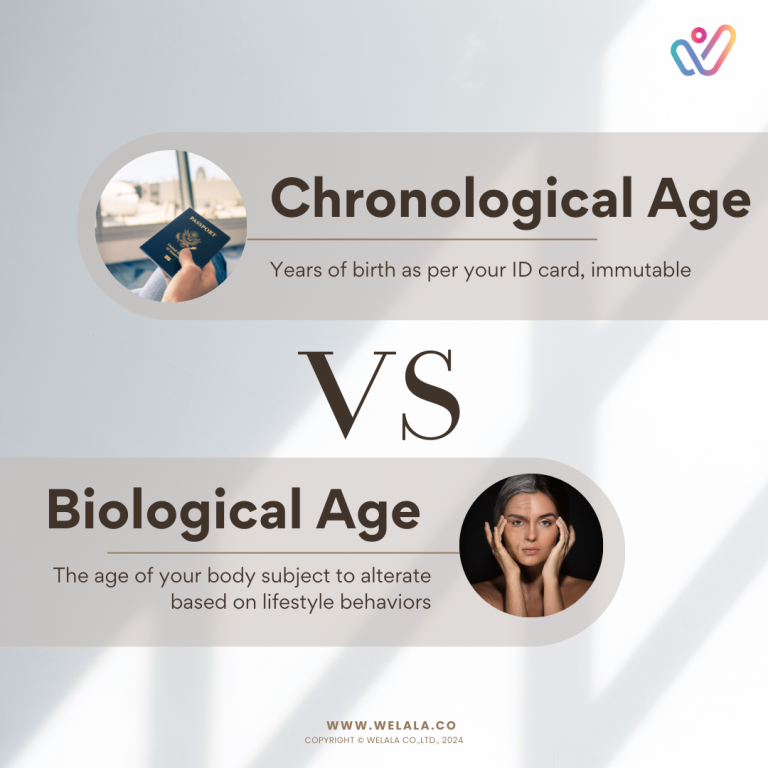
In the intricate dance of genetics and health, the conventional belief that our DNA dictates our destiny is undergoing a transformative shift. Epigenetic aging, a groundbreaking concept, is revealing that our biological age can be reversed. This blog explores the potential of understanding and influencing DNA methylation changes through the lens of GenomAGE, paving the way for lifestyle modifications that not only reduce the risk of diseases but also defy the conventional boundaries of aging.
Unlocking the Potential of Epigenetic Aging:
The notion that epigenetic aging can be reversed heralds a new era in personalized health management. With tools like GenomAGE providing insights into DNA methylation changes, individuals can actively engage in lifestyle modifications tailored to their unique genetic makeup. Armed with this knowledge, the prospect of reducing the risk of diseases and slowing down the aging process becomes not just a possibility but a tangible reality.
Innovative Studies and Promising Findings:
The forefront of scientific exploration is witnessing innovative studies focused on unraveling the mechanisms capable of reducing biological age. A standout example is the TRIIM trial, where researchers observed a remarkable average epigenetic age reduction of 1.5 years after just one year of treatment. By targeting the thymus, an organ crucial to immune function, they activated protective immunological changes, offering a glimpse into the potential of reversing biological age through targeted interventions.
Lifestyle Tips for a Younger Biological Age:
Our epigenetic marks, while more stable in adulthood, remain dynamic and modifiable through lifestyle choices. The profound impact of lifestyle on epigenetic effects throughout a lifespan is becoming increasingly evident. Intermittent fasting, a known stressor, has been shown to activate genes responsible for repairing DNA and protecting chromosomes. Incorporating dietary restrictions, particularly those inhibiting nutrient-sensing and inflammatory pathways, emerges as a powerful strategy promoting proteostasis, genome stability, and overall health.
Environmental Influence and Protective Measures:
The environment plays a pivotal role in shaping epigenetic tags and influencing disease susceptibility. Recent research sheds light on the concerning impact of air pollution altering methyl tags on DNA, increasing the risk for neurodegenerative diseases. On a positive note, B vitamins have been identified as potential protectors against the harmful effects of pollution. These findings underscore the interconnectedness of our environment, lifestyle, and epigenetic health.
Nutrigenomics and the Epigenetic Diet:
The emerging field of nutrigenomics explores the dynamic interplay between food and epigenetics. Dietary choices have been shown to modify epigenetic tags significantly. For instance, a high-fat, low-carb diet may influence chromatin, improving mental abilities through HDAC inhibitors. Research indicates that compounds in our diet can protect against cancer by adjusting methyl marks on critical genes. The evolving understanding of nutrigenomics paves the way for an epigenetic diet, guiding individuals toward optimal food regimens tailored to their unique epigenetic profiles.
As science unravels the complexities of epigenetic aging, the narrative shifts from a deterministic view of genetics to a proactive approach in managing health. Armed with tools like GenomAGE and insights from innovative studies, individuals have the power to shape their biological age and reduce the risk of diseases. The journey towards a younger biological age is not just a scientific possibility but a call to action for embracing personalized lifestyle modifications that transcend the conventional boundaries of aging.
References:
1. Fabbri, L., & Drazen, J. M. (2014). Ageing and the border between health and disease. Ageing Research Reviews, 31(1), 1332-1342.
2. Vijg, J., Ville, M., Sambrova, M., Wouters, P., & van der Meer, M. (2023). Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nature Medicine, 29(4), 602-610.
3. López-Otín, C. J., & Tang, C. (2018). The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. New England Journal of Medicine, 378(5), 465-475.
4. National Institute on Aging (NIA). (2019). Geroscience: The intersection of basic aging biology, chronic disease, and health. National Institute on Aging.
5. Franceschi, D., Baccarelli, C., Ciccia, M., & Campisi, J. (2018). The continuum of aging and age-related diseases: common mechanisms but different rates. Frontiers in Molecular Biosciences, 10, 61.
Citations:
[1] https://erj.ersjournals.com/content/44/5/1332
[2] https://www.nature.com/articles/s41591-023-02296-6
[3] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4817738/
[4] https://www.nia.nih.gov/research/dab/geroscience-intersection-basic-aging-biology-chronic-disease-and-health
[5] https://www.frontiersin.org/articles/10.3389/fmed.2018.00061