ONCO-PDO by Welala brings the most cost-effective outcome for cancer treatment in all stages of disease progression.
Carefully cryo preserve your biopsy during transporation
Tested with 100+ chemo / immuno / targeted drugs
Certified laboratory-based results
Contact our sales team to plan with your surgeon
Biopsy tissue will be collected and pickup at your hospital
Wait for 14 days to receive test result
Our scientists and oncologists will help you understand the results to choose the right medicine to treate your cancer
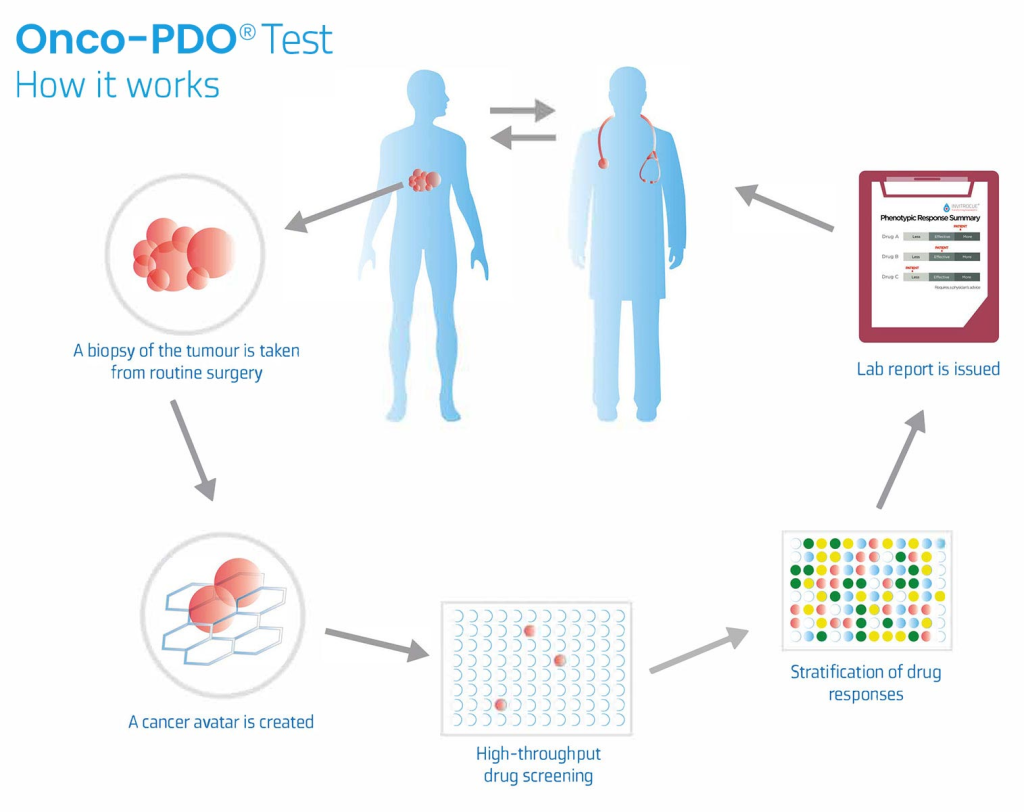
Onco-PDOTM test offers a strategic approach in targeting and personalizing your cancer treatment. A sample of your tumor is taken during surgery or routine biopsies and grown in the laboratory. The cells from this tissue sample are grown in a 3-dimensional environment like human body, which form complex structures called PDOs (Patient-Derived Organoids)
These PDOs are then tested against standard-of-care chemotherapeutic drugs (as recommended by your physician) to determine the response of Onco-PDOs under laboratory conditions. The studied response is then drafted as a Onco-PDOTM report and provided to your physician.#.
# Response data in the in vitro Onco-PDO® test may not always be reflective of patient-specific response due to additional physiological factors.
The test will provide an overview of how the cells extracted from your own tumor respond to a panel of standard chemotherapeutic drugs under laboratory conditions. This additional information from Onco-PDOTM report can provide an insight to your physician in understanding your tumor’s response to the drugs under laboratory conditions.
To date, this test has been done for breast, ovarian, lung, colorectal, pancreatic, gastric cancer as well as head & neck cancers (Asia only); we are in the process of optimizing the test for other cancer types, such as prostate. The test is intended for patients who will be undergoing chemotherapy and is especially useful for relapsed patients who have not shown response to first line therapy.
This test has been developed in collaboration with A*STAR’s Genome Institute of Singapore, a world-leading research institution in functional genomics and integrative biology. The technology supporting the test has been published in peer-reviewed journals like Nature Communications and Nature Medicine.
Once your samples are received for testing, results are typically available in 2-3 weeks to your physician*.
* There is no guarantee that a tumour model can be successfully developed from the sample taken to complete the testing, due to potential sample contamination, low cell number / viability or other limitations with the sample collected.
It is best to talk to your doctor^.
^ The Onco-PDO® Testing Service is not a substitute for visits to a physician. The information in these reports should not be used independently to determine or adjust any treatment plan. Many factors besides the information covered in these reports can influence how a patient responds to a drug.
Tests for patients in Europe are performed in our laboratory in Munich, Germany with the highest standards of laboratory practice. Tests for patients in Asia are performed in our laboratory in Singapore.
Patients are typically treated with drugs demonstrated to be effective from studies of the general population. Should the first treatment not work, oncologist would move on to the next best option. This trial-and-error approach means that while a patient’s particular cancer may be unique, their therapy is likely standardized. However, Welala’s advances in three-dimensional organoid technology have led to the development of the Onco-PDOTM (Patient-Derived Organoids) test that allows physicians to individualize treatment more effectively.
Onco-PDO™ test offers a strategic approach in targeting and personalizing the cancer treatment by creating a clinically relevant predictive in vitro model that quantifies PDOs response to standard chemotherapeutics.
A sample of the tumor is taken during surgery or routine biopsies and grown in the laboratory. The cells from this tissue sample are grown in a 3-dimensional environment like human body, which form complex structures called, PDOs.
These Onco-PDOs are then tested against standard-of-care chemotherapeutic drugs (as recommended by the physician) to study their response under laboratory conditions. The studied response is then drafted as a Onco-PDO™ report and provided to the physician.
A portion of a patient’s solid tumor (2-3 core biopsies) is mechanically/enzymatically disaggregated and established in primary culture within multi-well plates as organoids.
• These organoids are treated with chemotherapeutic agents (at Cmax). Cmax is the maximum serum concentration that a drug achieves in the body after the drug has been administrated, based on published literature.
• Following 72-96h of the drug treatment, we perform biochemical assays to determine the viable cells in comparison to a control (without treatment).
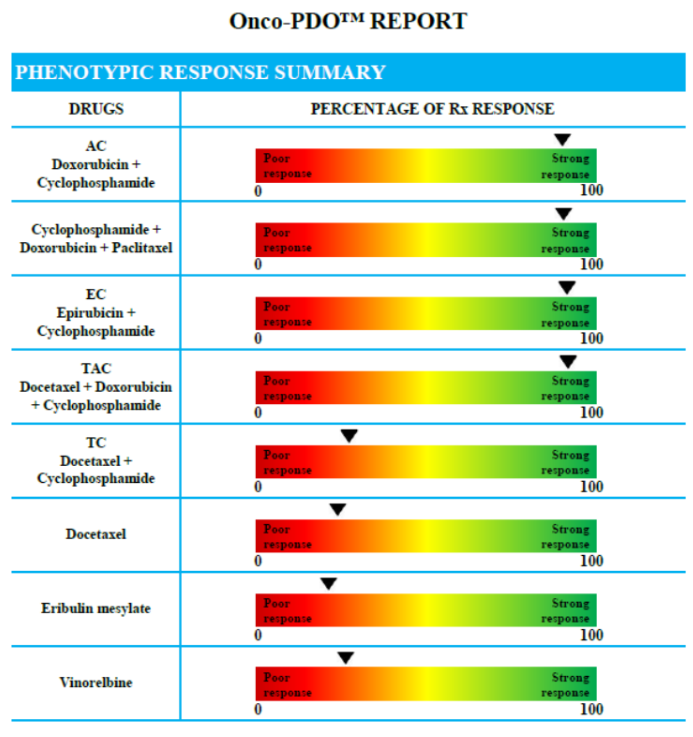
• The report illustrates the response of PDOs to the drugs under laboratory conditions and might assist the oncologist to make a more informed decision for each individual patient.
This test provides three key clinical advantages:
• Fast turnaround: The test report is delivered within 3 weeks to ensure continuity of clinical care without delaying treatment.
• Comprehensive: The test can include all standard-of-care chemotherapeutics to ensure the most comprehensive look at the patient’s potential treatment options.
• Actionable: The Onco-PDO™ report might help oncologist to make informed treatment decisions by identifying relevant and actionable findings based upon the response of the patient’s cancer cells to chemotherapeutics in the laboratory.
Next-Generation Sequencing (NGS) analyses cancer specimens for genomic alterations known to be relevant in cancer. The test results can only be used to suggest targeted therapies that are available or associated with the identified genomic alterations, based on published studies of the general population. By comparison, Onco-PDO™ test is a clinically relevant, real-time modelling and in vitro testing service that illustrates how individual patient’s own tumor cells respond to standard-of-care chemotherapies and targeted therapeutic agents.
The test is for patients who need chemotherapy, and most useful for patients with advanced cancer or relapsed patients who have not responded well to first line therapy. First line therapies are usually subjected to strict regulatory guidelines, and oncologists do not have much leeway to choose drugs other than standard-of-care drugs for their patients. For relapsed patients, oncologists have wider choice of drugs, and time is of essence for the patients as the body cannot afford to undergo any unnecessary round of chemotherapy, making every data point that could aid clinical decision-making even more valuable. Our hope is that one day the Onco-PDO™ test will become a standard-of-care test that can screen drugs for first line therapy as well.
To date, this test has been done for breast, ovarian, lung, colorectal, pancreatic, gastric cancers as well as head & neck cancers (Asia only); we are in the process of optimizing the test for other cancer types, such as prostate.
The test report will provide the following information:
• An overview of how the patient’s cancer cells have responded to the panel of standard-of-care chemotherapeutics in the laboratory.
Each individual report is specially designed to clarify the results for the ordering physician and aid in their treatment decision making.
Once your patient’s sample is received for testing, results should be available in 2-3 weeks.
We require fresh tumor tissue from surgery or biopsy, which are needed to be transported in chilled transport media to the laboratory within 24 hours. Formalin-fixed paraffin-embedded tumor tissue cannot be used.
Either a minimum of 1 cm³ of tumor tissue or 2-3 core needle biopsies are required. Anything less may risk the test failing to achieve completion.
Tests for patients in Europe are performed in our laboratory in Munich, Germany with the highest standards of laboratory practice. Tests for patients in Asia are performed in our laboratory in Singapore.